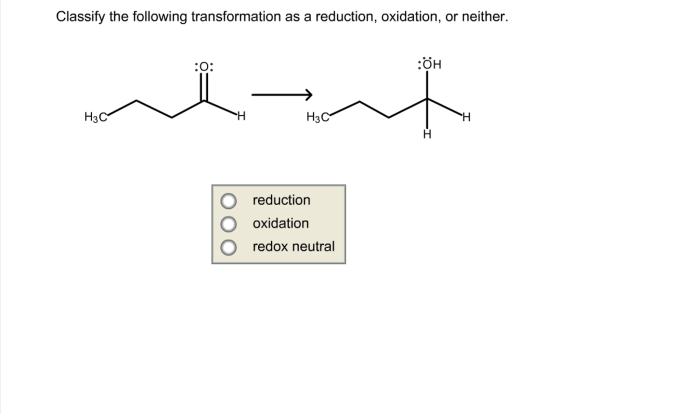
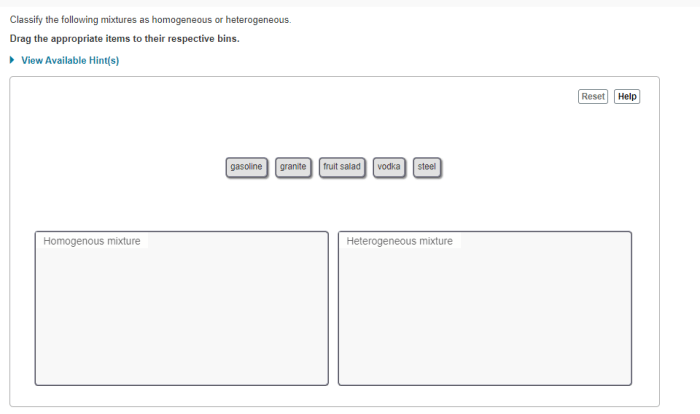
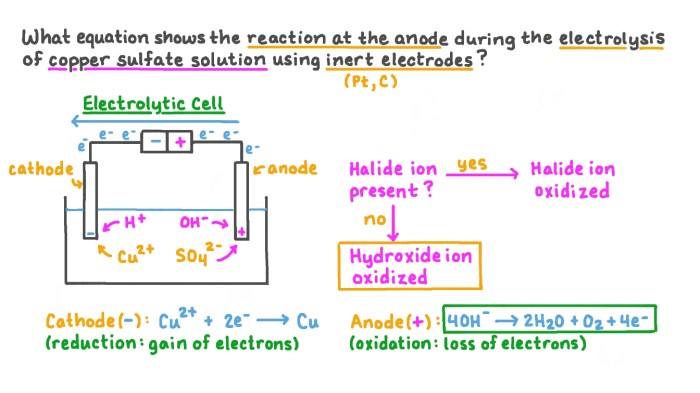
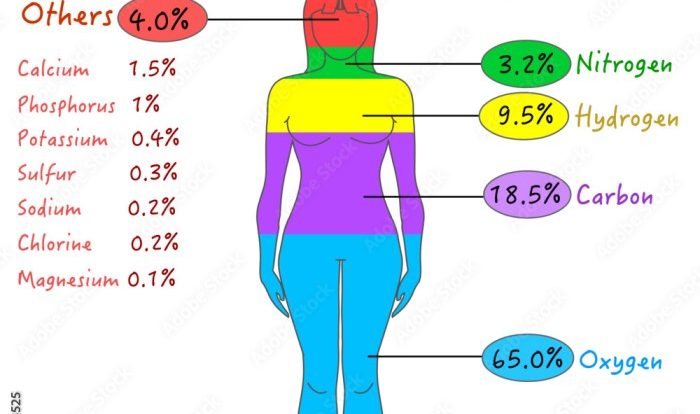
Prepare to excel in your UGA CHEM 1211 Lab Practical with this comprehensive guide. Covering essential topics and concepts, we’ll navigate you through the practical’s format and procedures, ensuring you master the skills for accurate data analysis and report writing.
Safety is paramount, so we’ll emphasize lab safety protocols to ensure a safe and successful learning experience.
Lab Practical Overview

The lab practical is a comprehensive assessment designed to evaluate your understanding of the fundamental concepts and techniques covered in UGA CHEM 1211 laboratory.
The practical will consist of a series of experiments and questions that will test your ability to:
- Apply laboratory safety protocols
- Perform basic laboratory techniques
- Analyze and interpret experimental data
- Draw conclusions based on your findings
Topics and Concepts Covered, Uga chem 1211 lab practical
The practical will cover the following topics and concepts:
- Laboratory safety
- Measurement and uncertainty
- Stoichiometry
- Gas laws
- Thermochemistry
- Equilibrium
- Acids and bases
- Electrochemistry
Experimental Procedures: Uga Chem 1211 Lab Practical
In this section, we will provide step-by-step instructions for each experiment in the lab practical. These procedures will include detailed information on materials, equipment, and safety precautions to ensure your safety and success during the lab.
Materials and Equipment
Before beginning any experiment, it is crucial to gather all the necessary materials and equipment. These may include glassware, chemicals, instruments, and personal protective equipment (PPE). Refer to the lab manual or instructions provided for a complete list of materials required for each experiment.
Safety Precautions
Safety is paramount in any laboratory setting. Prior to starting an experiment, familiarize yourself with the potential hazards associated with the materials and procedures involved. Wear appropriate PPE, including lab coat, gloves, and safety glasses, as specified in the lab manual or by the instructor.
Always handle chemicals with care, avoid spills and contact with skin or eyes, and dispose of waste properly.
Step-by-Step Procedures
Follow the step-by-step procedures Artikeld in the lab manual or instructions carefully. These procedures will guide you through the experiment, from setting up the apparatus to collecting and analyzing data. Read each step thoroughly before proceeding, and do not hesitate to ask for clarification if needed.
Data Collection and Analysis
Once you have completed the experiment, you will need to collect and analyze the data you have gathered. This may involve recording observations, taking measurements, and performing calculations. Use appropriate techniques to ensure the accuracy and reliability of your data.
The lab manual or instructions will provide guidance on how to analyze and interpret your results.
The UGA Chem 1211 lab practical is a breeze if you prepare well. Once you’ve got that down, you can check out the sod year 6 exam questions to get a head start on your studies for that as well.
That way, you can kill two birds with one stone and make the most of your study time. Returning to the UGA Chem 1211 lab practical, remember to review the lab manual and practice the experiments beforehand.
Data Analysis and Interpretation

The data collected during the practical will provide valuable insights into the chemical reactions and principles studied. Analyzing and interpreting this data is crucial to draw meaningful conclusions and understand the experimental outcomes.
To begin, carefully examine the raw data and identify any outliers or inconsistencies. Outliers can arise due to experimental errors or measurement uncertainties. If outliers are present, consider excluding them from the analysis or investigate their potential causes.
Calculating Results
Next, calculate the results based on the experimental data. This may involve using formulas, equations, or statistical methods. Ensure that the calculations are accurate and follow the specified procedures.
Drawing Conclusions
Once the results are calculated, draw conclusions based on the observed trends and patterns. Consider the following:
- Compare your results to the expected outcomes or theoretical predictions.
- Identify any significant differences or deviations from the expected values.
- Discuss the possible reasons for these differences, considering experimental errors, limitations, or other factors.
- Formulate a clear and concise statement summarizing the main findings of the experiment.
Safety Considerations
Maintaining a safe environment in the laboratory is paramount for your well-being and the integrity of the experiments conducted. Adhering to the established safety guidelines ensures a productive and hazard-free learning experience.
Before engaging in any laboratory activities, it is crucial to familiarize yourself with the specific safety protocols Artikeld for the course. These protocols address the proper handling of chemicals, equipment, and waste disposal.
Chemical Handling
- Always wear appropriate personal protective equipment (PPE), including gloves, lab coats, and safety glasses.
- Handle chemicals with care, avoiding direct contact with skin or eyes.
- Never mix chemicals unless specifically instructed to do so.
- Dispose of chemicals properly according to the designated waste containers.
Equipment Handling
- Inspect equipment before use and report any damage or malfunction to the instructor.
- Use equipment only for its intended purpose.
- Handle glassware with caution, as broken glass can cause serious injuries.
- Never look directly into the opening of a test tube or reaction vessel.
Waste Disposal
- Dispose of chemical waste in designated containers labeled for specific types of waste.
- Do not pour chemicals down the sink or dispose of them in the trash.
- Follow the instructions provided by your instructor regarding the proper disposal of sharps and biological materials.
Study Resources

Preparing for your lab practical is essential to ensure success. Here are some resources to help you excel:
Textbooks
The assigned textbook for this course provides comprehensive coverage of the material covered in the lab practical. Make sure to thoroughly review the relevant chapters and practice the example problems.
Online Materials
- Course website:The course website often contains additional resources, such as practice problems, lecture notes, and videos.
- Online learning platforms:Many online learning platforms offer interactive simulations, quizzes, and practice tests that can supplement your textbook learning.
- YouTube videos:Search for videos related to the lab practical topics. Visual demonstrations can enhance your understanding.
Practice Problems
Solving practice problems is crucial for testing your understanding and identifying areas where you need further review. Your textbook and online materials will provide plenty of practice problems to work through.
FAQ Resource
What is the purpose of the UGA CHEM 1211 Lab Practical?
The lab practical assesses your understanding of fundamental chemistry concepts and your ability to apply them in a laboratory setting.
What topics are covered in the lab practical?
The practical covers a range of chemistry topics, including chemical reactions, stoichiometry, and equilibrium.
How do I prepare for the lab practical?
Thoroughly review the course material, practice solving problems, and utilize the study resources provided in this guide.
What safety precautions should I follow during the lab practical?
Always wear appropriate safety gear, follow the instructions carefully, and be aware of potential hazards.