Classify the transformation as a reduction oxidation or neither – In chemistry, classifying chemical transformations as reduction, oxidation, or neither is crucial for understanding electron transfer processes and predicting reaction outcomes. This article delves into the concept of reduction and oxidation reactions, providing a comprehensive guide to identifying and classifying chemical transformations.
By exploring the role of electron transfer and providing practical examples, we aim to enhance your understanding of this fundamental aspect of chemistry.
Types of Transformations: Classify The Transformation As A Reduction Oxidation Or Neither
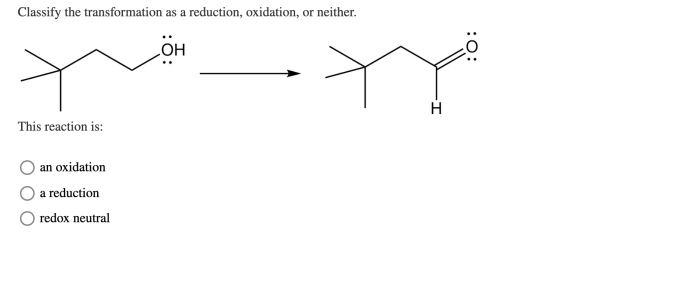
Transformations in chemistry involve changes in the electronic structure of atoms or molecules. These changes can be classified as reduction, oxidation, or neither, based on the movement of electrons.
Reduction, Classify the transformation as a reduction oxidation or neither
Reduction occurs when a species gains electrons, resulting in a decrease in its oxidation state. The species becomes more negative in charge.
Oxidation
Oxidation occurs when a species loses electrons, resulting in an increase in its oxidation state. The species becomes more positive in charge.
Neither Reduction nor Oxidation
Some transformations do not involve any change in oxidation state. These transformations are classified as neither reduction nor oxidation.
Identifying Reduction and Oxidation
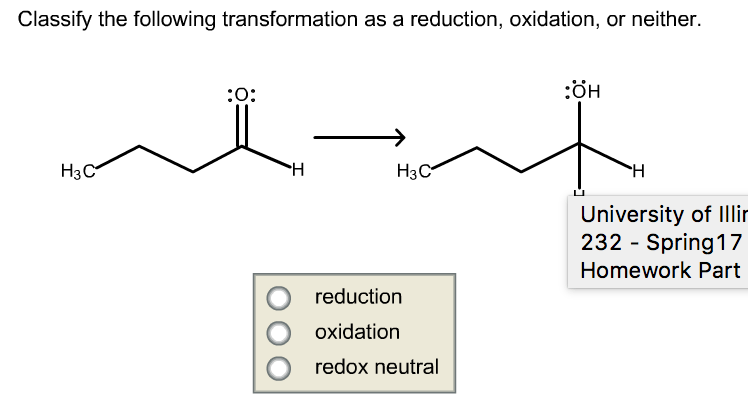
To identify whether a transformation is a reduction, oxidation, or neither, follow these steps:
- Determine the oxidation state of each element in the reactants and products.
- Compare the oxidation states of each element in the reactants and products.
- If the oxidation state of an element decreases, it has undergone reduction.
- If the oxidation state of an element increases, it has undergone oxidation.
- If there is no change in oxidation state for any element, the transformation is neither reduction nor oxidation.
Examples of Transformations
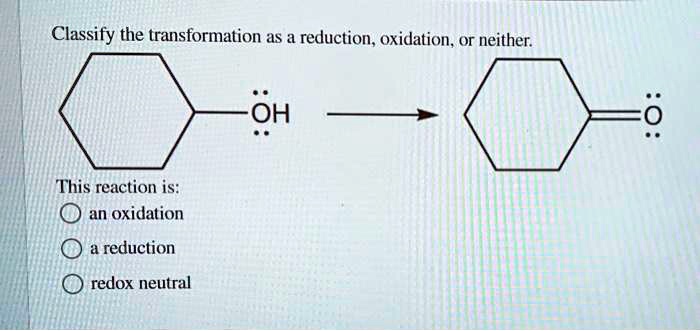
| Transformation | Type | Chemical Equation |
|---|---|---|
| Na + Cl → NaCl | Oxidation | Na0 + Cl0 → Na+ + Cl– |
| Fe2O3 + 3CO → 2Fe + 3CO2 | Reduction | Fe3+ + 3C0 → 2Fe0 + 3C4+ |
| HCl + NaOH → NaCl + H2O | Neither | H+ + Cl– + Na+ + OH– → Na+ + Cl– + H2O |
Applications of Classification
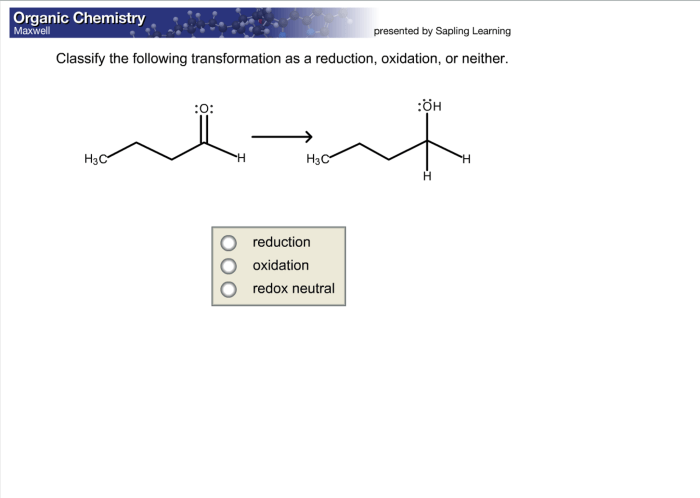
Classifying transformations as reduction, oxidation, or neither is important in chemistry because it allows us to:
- Predict the products of reactions.
- Design new materials with specific properties.
- Understand the mechanisms of chemical reactions.
FAQs
What is the difference between reduction and oxidation?
Reduction involves gaining electrons, while oxidation involves losing electrons.
How can I identify whether a transformation is a reduction or oxidation?
Look for changes in oxidation states of the reactants and products.
Why is classifying transformations important?
It helps predict reaction outcomes, design materials, and understand electron transfer processes.